What is Stoichiometry?
- In a chemical reaction, different reactants react together in different quantities and generate products.
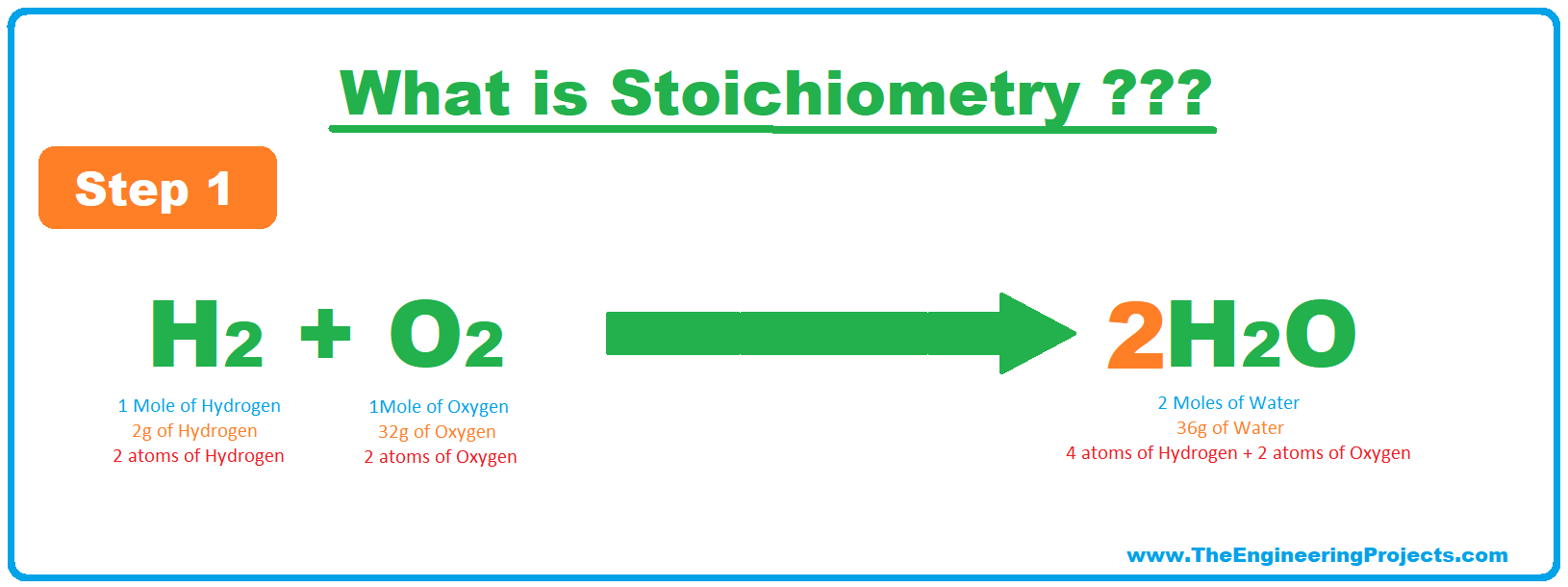
- For example, in the below chemical reaction, Hydrogen & Oxygen are reactants and they are producing water as a result of an exothermic reaction:
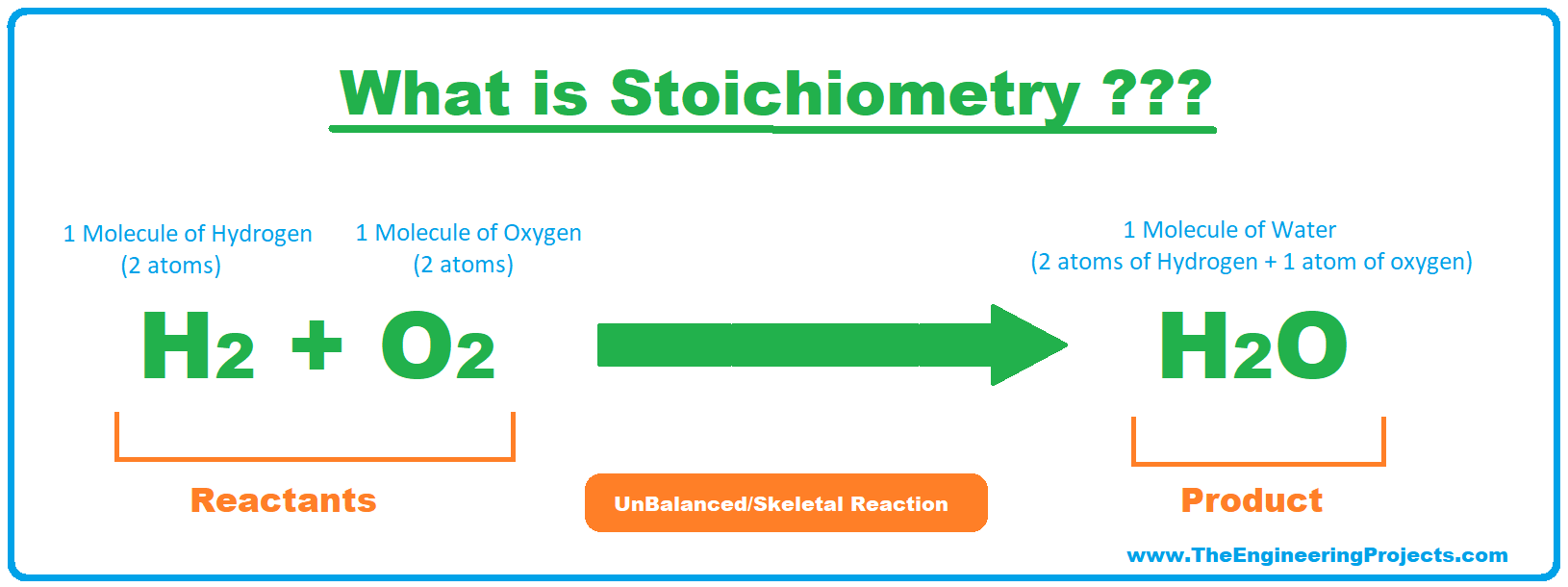
- As you can see in the above reaction,
- Reactants: 2 atoms of hydrogen + 2 atoms of oxygen
- Product: 2 atoms of hydrogen + 1 atom of oxygen.
- So clearly, there's a molecular difference between reactants & products and according to the Law of Conversation of mass:
- In simple words, the molecular mass of reactants must be equal to that of products and if it's not the case, then such reactions are called UnBalanced Reactions.
UnBalanced Reactions
- Unbalanced Reactions are also called Skeletal Reactions and provide information only about the type of ingredients & Products used in a chemical reaction.
- It doesn't give any information about the quantity of the reactants or the products.
Balanced Reactions
- A chemical reaction, which strictly follows the law of conservation of mass i.e. the molecular mass of reactants must be equal to that of products is called Balanced Reaction.
- For balancing a chemical reaction, numerical coefficients are used and are placed on the left side of the entity.
- Now let's balance the above unbalaced reaction to understand it completely, balanced reaction is shown in the below figure:
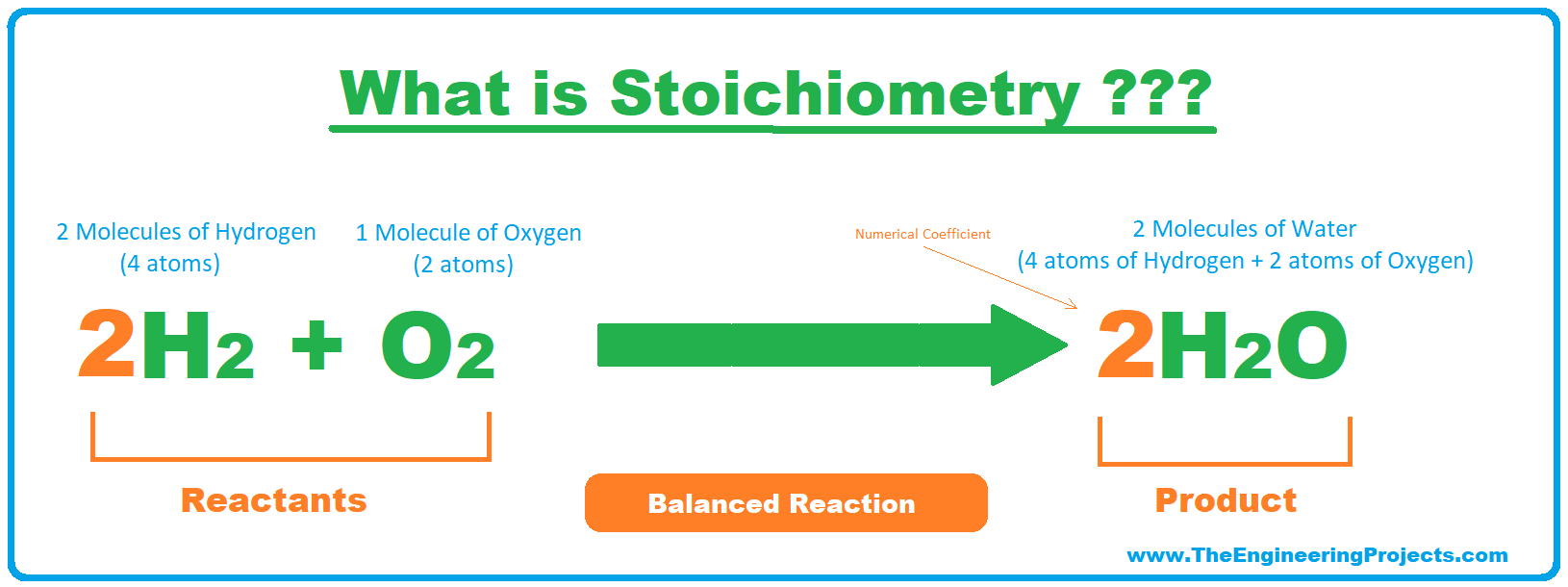
- As you can see in the above figure, I have used Numerical values called Coefficients to balance the equation.
- Now, in this balanced reaction, we have an equal number of atoms in reactants and products, i.e.:
- Reactants: 4 atoms of Hydrogen + 2 atoms of Oxygen.
- Products: 4 atoms of Hydrogen + 2 atoms of Oxygen.
- These coefficients are simply multiplied by the number of atoms.
- This technique of balancing a chemical reaction is called Stoichiometry.
Advantages of Balancing a Chemical Reaction
- As we have seen in the above water reaction, once we have balanced the reaction, it got clear that we need 2 moles(molecules) of Hydrogen and 1 mole(molecule) of oxygen, if we want to produce 2 moles(molecules) of water.
- So, with the help of a balanced equation, a scientist can easily calculate the number of ingredients for producing a certain amount of product.
Stoichiometry Definition
- Stoichiometry is a set of mathematical techniques, used for determining a quantitative relationship between reactants and products in a chemical equation/reaction.
- The term stoichiometry has been derived from the two Greek words, the first one is “Stiochos” which means elements and the second one is “metry” which means measuring, so the word collectively means "Measuring Elements".
- It was coined by Jeremias Benjamin in 1972, in the first volume of his book Richter's Stoichiometry also known as the Art of Measuring the Chemical Elements.
Stoichiometric Coefficients:
- Coefficients are the whole numbers, written in front of the elemental symbols in the equation, indicating the number of moles or the number of molecules.
- If there is no coefficient in front of a symbol, then 1 is assumed to be the coefficient.
Why do we need stoichiometry?
- We need stoichiometry for two reasons, mentioned as follows:
- For balancing a chemical reaction.
- For conversions i.e. grams to moles & moles to grams.
Balancing a Chemical Reaction
- There are no fixed rules for balancing a chemical reaction, it depends more on your analytical skills, but there are few tricks.
- While balancing a chemical reaction, always balance individual elements one by one, as in a water reaction, we first balanced oxygen and then hydrogen.
- First balance that element, which has the least occurrences in the reaction, as for water oxygen atoms appeared 3 times, while hydrogen atoms were 4 times.
- Try to balance single elements first, as balancing elements from a compound is bit difficult.
- But again as I said earlier, these are few tricks to ease the process, but it mainly depends on analytical skills and practice.
- Your equation is said to be balanced when it will have an equal number of atoms on both sides i.e. obeying the Law of conservation of mass.
- So, let's balance few reactions to understand How it works:
Stoichiometry Example1: Water Reaction
- I have already shown both unbalanced & balanced equations of water but now let's have a look at the steps taken to balance it.
- So, we have an unbalanced equation as shown in the below figure:
- As you can see in the above figure, we have an unbalanced equation, so we need to analyze, which element can be balanced easily.
- Clearly, we are using 2 atoms of oxygen in reactants but the product contains only 1 atom.
- So, we need to multiply the product by 2 so that, we could balance oxygen items, as shown in the below figure:
- We have balanced oxygen items in the above equation, but now in reactants, we are using 2 hydrogen atoms but in the product, we have 4 atoms.
- So, in order to balance hydrogen atoms, we need to multiply hydrogen reactant with 2 as well, as shown in the below figure:

- Now we have an equal number of atoms(of both hydrogen & oxygen) in reactants and product and we can say, we have a balanced equation now.
- So, if we want to produce 2 moles of water(36g molar mass), then we have to combine 2 moles of hydrogen(4g) and 1 mole of oxygen(32g).
Stoichiometry Example 2: Propane Reacts with Oxygen
- Now, lets have a look at another reaction, where Propane reacts with oxygen and generates carbon dioxide and water, as shown in the below figure:
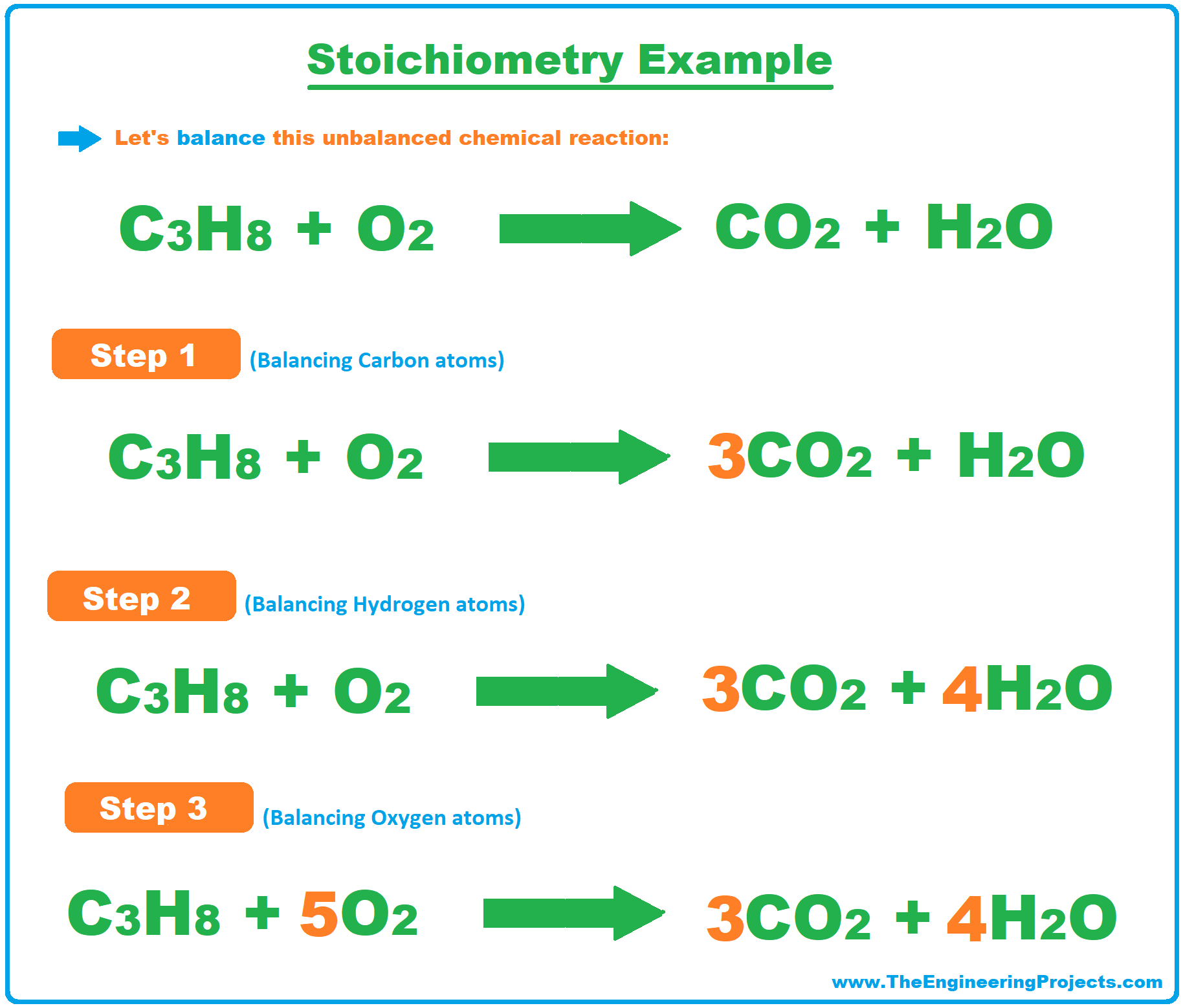
- As you can see in the Stoichiometry example, I have followed the above mentioned tricks.
- First of all, the point to notice is I have solved all elements individually, first Carbon, then Hydrogen and finally Oxygen.
- Moreover, I have Carbon first because it has appeared in two entities and was quite easy to balance.
- And in the last step, we got our balanced equation having an equal number of atoms on both sides of the reaction.
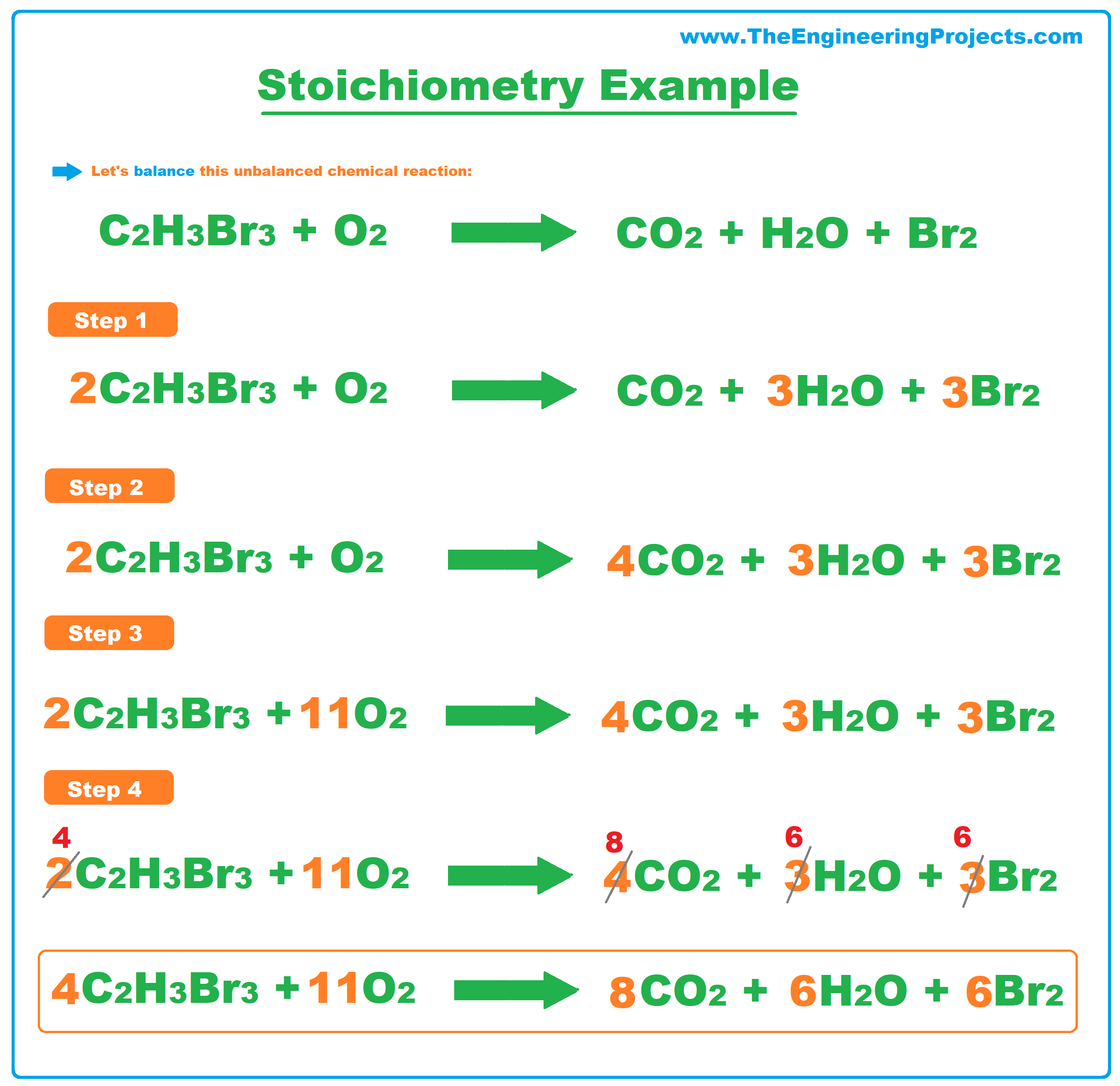
Stoichiometry Example 3
- Here's a quick example to show you that in complex equations, we may have to re-evaluate our coefficients, as shown in the below figure:

Unit Conversions using Stoichiometry
In the previous section, we have seen How to balance a chemical reaction and now we will discuss How to make unit conversions of chemical elements using these balanced equations.- Normally, the quantity of a chemical substance is measured using two different units, which are:
- Moles.
- Grams.
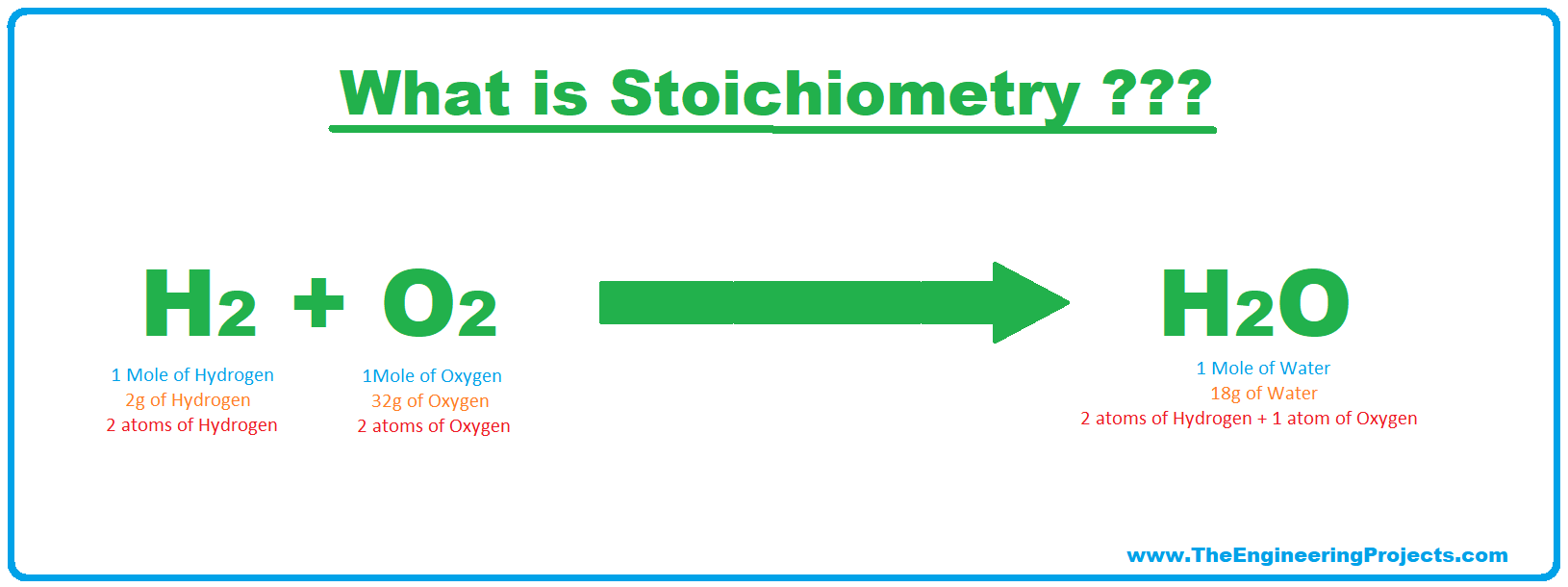
Moles Definition
- Mole is the SI unit for quantity/amount of a chemical substance and 1 mole of any substance contains 6.02 × 1023(Avogadro's no) atoms of that chemical substance.
- The term molecules and moles are used interchangeably, and if you ask me, mole is just the short form of the molecule.
- So generally, the number of molecules of a chemical substance, used in a chemical reaction is denoted by the unit called Mole(denoted as mol).
- As you can see in the below water reaction, 2 moles of Hydrogen are reacting with 1 mole of oxygen and producing 2 moles of water.

Grams Defnition
- When chemical substances are measured using their molar/molecular mass, then the Grams unit is used, denoted by g.
- Molar mass can be defined as the mass of one mole of a substance in grams.”
- Again let's understand it with water reaction:

- So, in the above reaction, I have displayed both moles and grams, so if you want to use the grams unit then we can say that:
- 4g of hydrogen is reacting with 32g of Oxygen and producing 36g of water.
- You must have noticed that we have an equal number of mass(in grams) on both sides of the equation as the balanced equation must obey Law of conservation of mass.
Moles Vs Grams
The following table shows the difference between Moles & Grams:| ESP32 Module Features and Technical Specs | ||||
|---|---|---|---|---|
| No. | Mole(mol) | Grams(g) | ||
| 1 | The Mole unit is used to count the number of entities(chemical substances) used. | Gram unit is used to measure the amount(molar mass) of chemical substance i.e. how much quantity is used? | ||
| 2 | Moles are normally integers | mass in grams could be a fraction. | ||
| 3 | Total moles of reactants may or may not be equal to that of products in a balanced chemical reaction. | The total mass(in grams) of reactants must be equal to that of products in a balanced chemical reaction. | ||
| 4 | Mole unit is used in theory mostly. | |||
Molar Ratio
- As we have seen, Stoichiometry is the knowledge of balancing the chemical equations.
- These chemical reactions are actually giving us the ratio between reactants and products.
- Let's understand this ratio with water reaction:

- So, in water reaction, we have a ratio of 2:1:2 between Hydrogen, oxygen and water respectively.
- As this ratio is between moles of the reactants and products, thus it's called the Molar ratio.
Conversions between Grams & Moles
There are four types of conversions that can be performed between these two units in stoichiometry, which are:- Moles-Moles Conversion
- Grams-Moles Conversion
- Grams-Grams Conversion
- Moles-Grams Conversion
Stoichiometry Problems
In stoichiometry we come across a number of numerical problems that ask for the following calculations;- Finding out the limiting reactant of a reaction.
- Calculating the actual yield of a reaction.
- Theoretical yield of a chemical reaction.
- Finding out the empirical formula of a compound after combustion analysis.
Find Limiting Reactant using Stoichiometry
Limiting reactant can be defined as:- A limiting reactant(also called a limiting agent) is a chemical substance/element, that takes part in a chemical reaction in a limited amount & controls the amount of product produced.
Stoichiometry Problem Statement:
During a chemical reaction 3.2 moles of N2 react with 5.4 moles of H2 to form NH3, how much amount of NH3 can be formed in the process? Which one is the limiting reactant? How of the excess reactant would be left over after the consumption of the limiting reactant? Solution:Step 1: Write the Balanced Equation of the chemical reaction:
- We will start by writing the balanced chemical equation for the above reaction
N2 (g) + 3H2 (g) ? 2NH3 (g)
Step 2: Determine the Limiting Reactant b/w N2 & H2
- We will now determine the amount of N2 consumed by H2.
- So, from above balanced equation, we have the molar ratio of 1:3:2 between nitrogen, hydrogen and ammonia respectively.
- So, we can find the limiting reactant as shown in the below figure:
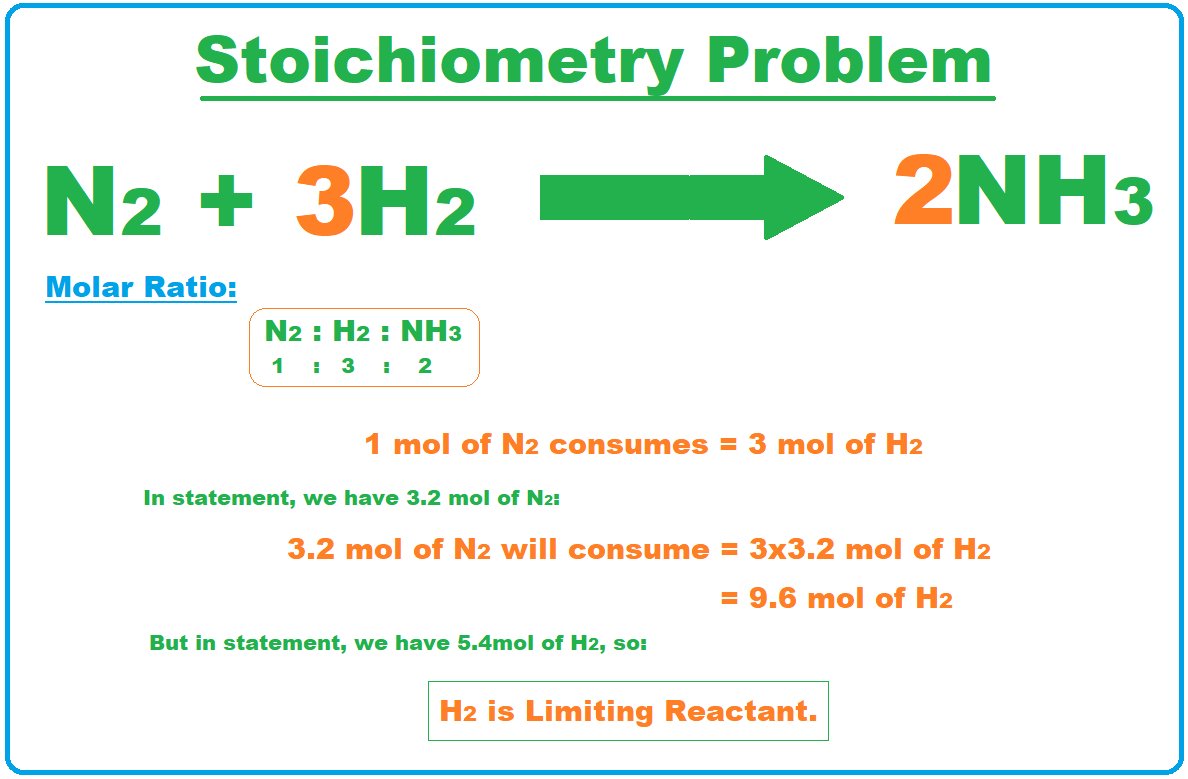
- As, we can see in the above calculations that 9.6 mol of H2 Is required to fully consume 3.2 mol of N2, but in the statement, we are only provided with 5.4 mol of H2, so clearly H2 is our limiting reactant here.
- Now, we will be calculating the total amount of N2 consumed with the provided 5.4 mol of H2, so:
3 mol of H2 consumes = 1 mol of N2
1 mol of H2 consumes = 1/3 mol of N2
5.4 mol of H2 consumes = 5.4 × 1 / 3 mol of N2 = 1.8 mol of N2.
Step 3: Determine maximum product produced by the limiting reactant
- Next, we need to find the amount of product, that we can produce using these reactants.
- As we already know that H2 is our limiting reactant, so simply we need to apply the molar ratio between H2 and NH3.
- For this, we can do the following calculation:
3 mol of H2 produces = 2 mol of NH3
1 mol of H2 will produce = 2/3 mol of NH3
5.4 mol of H2 will produce = 5.4 × 2 / 3 mol of NH3 = 3.6 mol of NH3
- So, 3.6 mol of ammonia is the maximum amount, that can be produced with the number of reactants given in the problem statement.
Step 4: Determine the amount of excess reactant
- If you want to determine the amount of N2 still left after the reaction, there is a simple formula for that i.e. number of moles given - number of moles used = amount of excess reactant left
- 3.2 mol - 1.8 mol = 1.4 mol of N2 are left after the consumption of the limiting reactant.
Step 5: Conclude the results
The conclusion can be written as;- H2 is the limiting reactant
- N2 is the excess reactant, so only 1.8 mol will be consumed and 1.4 mol will be left.
- 3.6 mol of NH3 can be produced with the reaction of 5.4 mol of H2 and 1.8 mol of N2.
Calculating Actual Yield and Theoretical Yield
First, let's have a look at their definitions:Yield Definition
- It is the amount of product formed during a chemical reaction.
Theoretical Yield
- The amount of stoichiometric product calculated on paper without any practical experimentation, we can also regard it as an estimated product.
Experimental/Actual Yield
- The actual amount of product formed during a chemical reaction on practical grounds.
- We don't have ideal conditions of temperature and pressure in the industry
- There is a loss of reactants in the process of transferring, mixing etc.
- Mechanical losses are always there which can never be ignored.
- An impure reactant can add to the agony of the procurement team as well
Percentage yield
- Percentage yield is the percentage ratio of actual/experiment yield to the theoretical yield.
- It can be mathematically written as:
% yield = actual yield / theoretical yield × 100
Why we use this concept? You must be thinking why we need this simple percentage in our lives, let me tell you why! It is extremely crucial to industries either chemical or pharmaceutical, they calculate the amount of profit or loss through this method. You might have faced the discontinuation of your favorite soap or shampoo or bubble gum in your life, it was all due to the decrease in the %age yield of the product that was not good enough to continue because when a product has a lesser profit margin there is no other choice than to discontinue it! Production is highly dependent on this concept regarding it to be a success factor for a product to rule the market. It’s time for a numerical problem to help you better understand the concept!PROBLEM STATEMENT
42 grams of hydrogen reacts with nitrogen to form 120g of ammonia, determine the percentage yield of the product formed during the reaction.Step1: Write the balanced chemical equation
N2 (g) + 3H2 (g) ? 2NH3 (g)
Step 2: Convert grams into moles
- As the quantities are given in grams, so we need to convert them to moles first. So,
Moles of H2= mass in grams / molar mass
= 42/2 = 21 moles
Step 3: Calculate NH3 Theoretical Yield
- Next, we need to calculate the moles of the product by comparing it with the reactant using a balanced chemical equation.
- So, the molar ratio between H2 & NH3 is 3:2, so:
3 mol of H2 produces = 2 mol of NH3
1 mol of H2 will produce = 2/3 mol of NH3
21 mol of H2 will produce = 21 x 2/3 mol of NH3 = 14 mol of NH3
Convert moles into grams because the actual yield has been given in grams and units of two quantities to be compared must be the same.Mass in gram of NH3= number of mole × molar mass = 14 × 17= 238g
Step 4: Calculate Percentage Yield
- Now, calculate the percentage yield by putting the values into the given formula:
Actual yield of NH3 = 120 grams
% yield = actual yield ÷ theoretical yield × 100
= 120 / 238 × 100
= 50.5% is the calculated percentage yield of NH3
This is how we calculate the %age yield of a chemical reaction.Calculating Empirical formula with Stoichiometry
As we are well aware of combustion, how and why it happens, it's time to solve the stoichiometric problems related to combustion analysis in which students are mostly asked to find empirical formulas. The empirical formula is different from the molecular formula, don't confuse both with each other here's why! “Empirical formula can be defined as the simplest ratio of atoms or elements present in a compound” Example: The molecular formula of benzene is C6H6 meanwhile the empirical formula is CH. Features of Empirical Formula:- Contains the most simplified ratio of the moles of elements making a compound
- It is simply a ratio, not an exact number or amount of atoms or molecules making a compound.
- Determined by weight percentages by converting them into grams.
- It is not commonly used in experimental schemes as compared to molecular formulas.
- It is the actual number of atoms or molecules forming the compound
- We calculate it after the calculation of the empirical formula
- It is a by-weight representation of every constituent particle making the molecule.
- It is commonly used in chemical reactions and stoichiometric calculations
- The molecular formula is always a multiple of the empirical formula; we can calculate empirical formula by a simple calculation at glance.
NUMERICAL PROBLEM
- A compound is composed of 52.14% of carbon, 34.7% oxygen and 13.13% of hydrogen by mass. Given that the molar mass of the compound is 138.204 g/ mole, Calculate The empirical formula and molecular formula.
Step 1: Convert percentages into grams
- Carbon = 52.14g
- Hydrogen =13.13g
- Oxygen=34.7g
Step 2: Convert grams into moles
- For carbon:
no of moles = mass in grams/ molar mass
52.14 = mole / 12 g per mole = 4.34 moles
- For hydrogen:
no of moles = mass in grams / molar mass = 13.13 / 1 = 13.02 moles
- For oxygen:
no of moles = mass in grams / molar mass = 34.73/ 16 = 2.17 moles
Step 3: Dividing with a common denominator
- Divide the number of moles of all 3 atoms with the lowest number of moles obtained:
Number of moles for Carbon =4.34 moles/ 2.17= 2
Number of moles of hydrogen= 13.13/2.17=6
Number of moles of oxygen = 2.17/2.17 =1
Step 4: Setup your Empirical formula
- C2H6O is our empirical formula
Step 5: Calculate the molecular formula
- Add up and calculate the atomic mass of each element
2C + 6 H + O
= 2×12 + 6× 1.008 + 16 = 46.08
Molar mass/mass in grams = 138/46 = 3
Multiply 3 with the empirical formula subscripts
So the molecular formula is C6H18O3
This is how we calculate the empirical formula, summarizing it;- Convert %age into grams
- Then convert grams into moles afterward
- Divide the moles with the least amount of moles obtained
- The digit obtained is the empirical formula!



 Chemistry
Chemistry syedzainnasir
syedzainnasir 0 Comments
0 Comments

















