Hello, friends today we will discuss the basic concept of chemistry it is our first tutorial series in which we will discuss:
- Atom
- Molecule
- Ion
- Molecular ion
Now in this article, we will discuss atoms. Its definitions, examples, properties, its evolutionary history, and also some important facts in the form of questions.
Atom
Definitions
A tiny particle that cannot be seen with a naked eye so-called atom.
Or
Atom is the lowest unit of matter and is often divided without the discharge of electrically charged particles.
Or
Atom is the introductory structure block of chemistry.
Examples
From molecule;
Hydrogen (H2)
- It has two atoms.
Nitrogen (N3)
- It has three atoms
From elements;
Helium(He)
- It has two electron
Properties
We discuss different properties of atoms like:
Atomic no:
The no of protons present in the nucleus of an element is called atomic number Or nuclear charge no.
. Its symbol is Z.
- Always a whole no
- No effect of neurons on atomic no.
- Always give a smaller value than atomic mass.
Examples
- Carbon has 6 protons in its nucleus so its atomic no is Z=6
- Sodium has 11 protons .so Z= 11
Atomic mass
The sum of the numbers of neutrons and protons in the nucleus of an atom is called atomic mass or mass no.
- It is represented by A and calculated as A=Z+n
- Where n is no of neutrons and Z is no of protons
- It always is greater than atomic no.
- It is affected by the addition of neutrons.
Example
Sodium has 11 electrons = 11 protons and 12 neutrons in an atom. So its mass no is
A= 11+12= 23
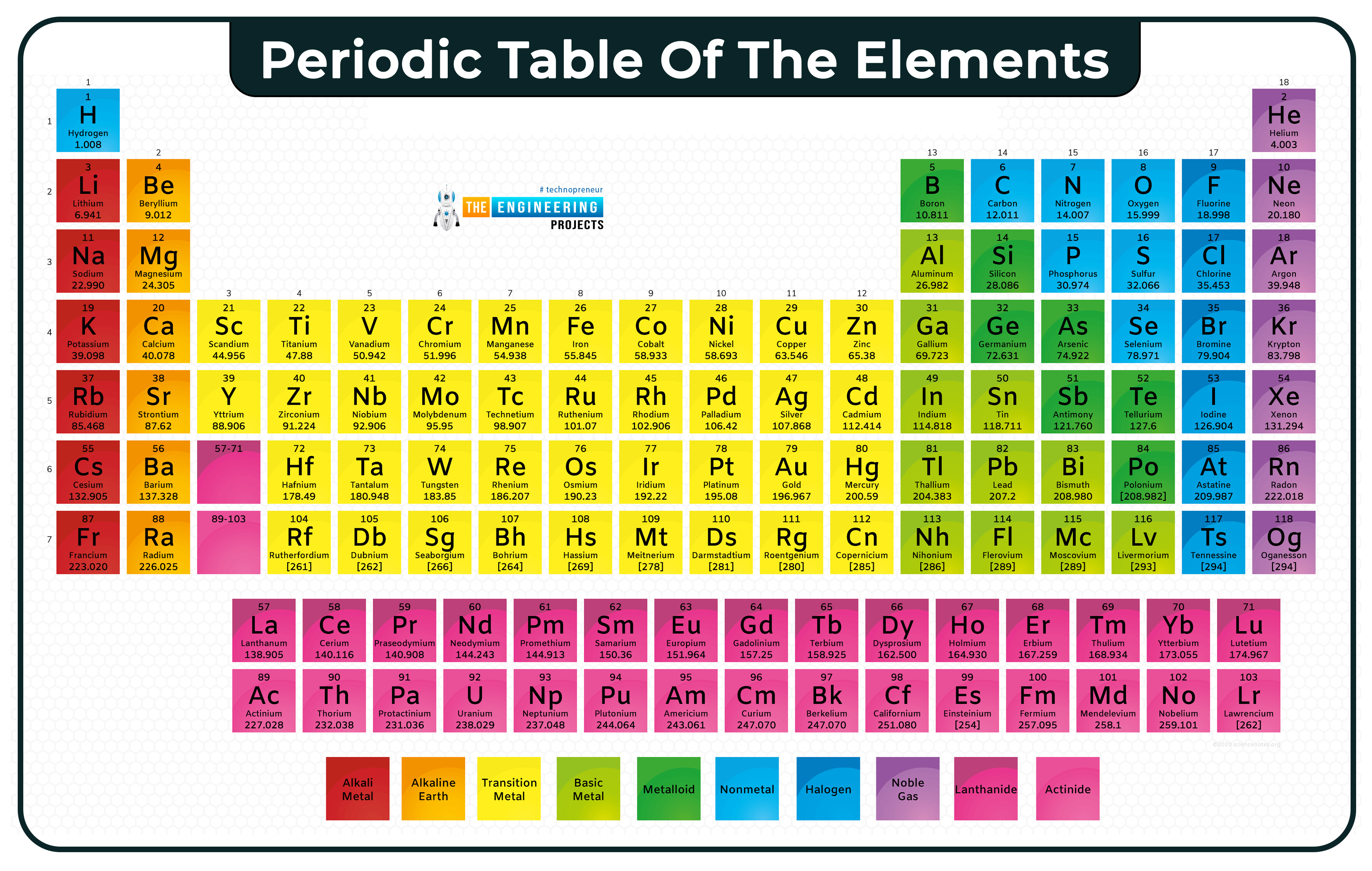
Periodic table
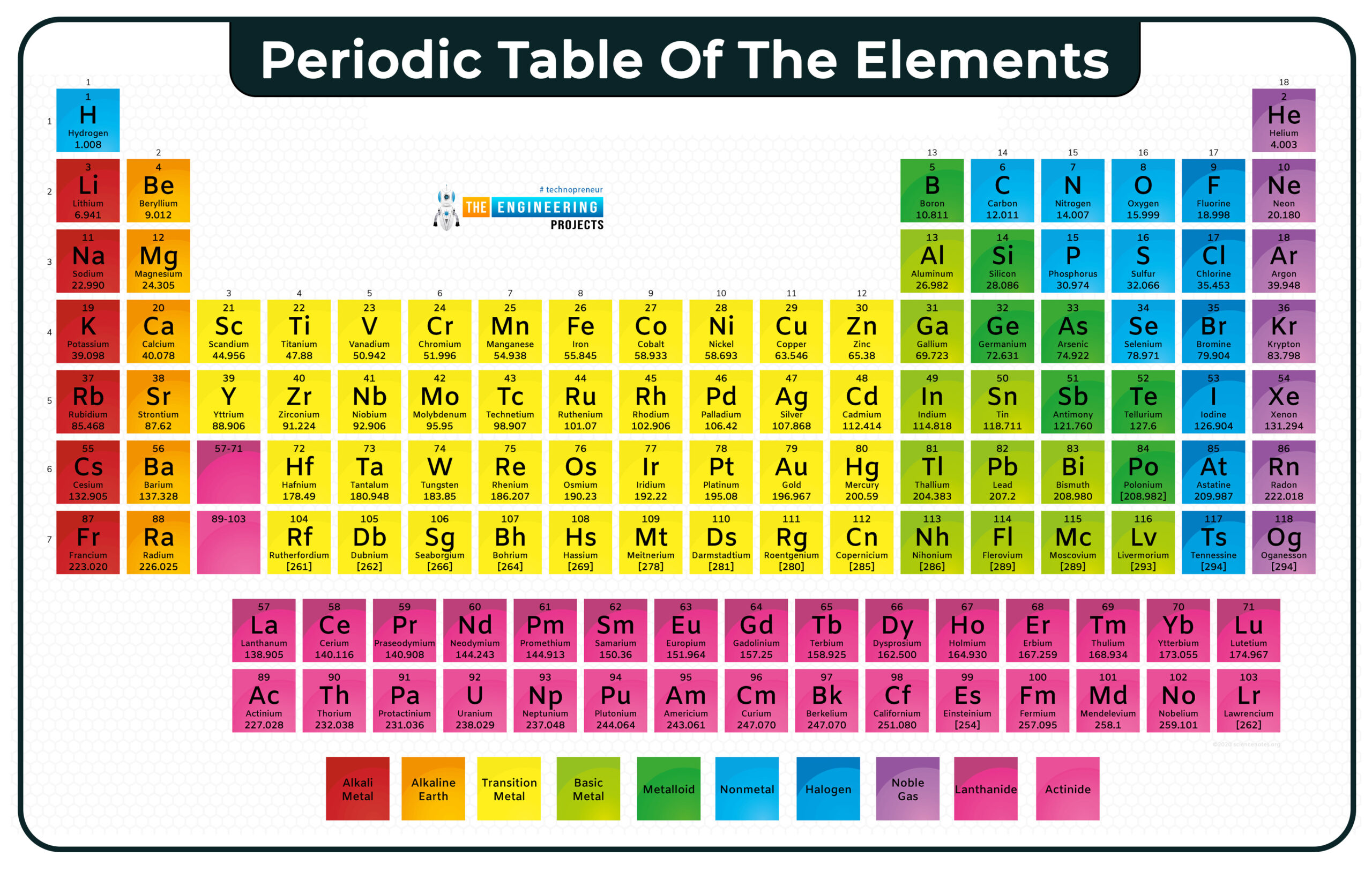
In this table, we can see atomic numbers, atomic mass, and symbols of atoms. Above the symbol is atomic no and below is the atomic mass.
Atomic size.
- We can not measure the size of an atom because atoms have no boundaries and they are very tiny, so can assume spherically
- It decreases if the atomic no is decreased from left to right in a period.
- In groups, atomic size gradually increases from top to bottom.
Atomic radius
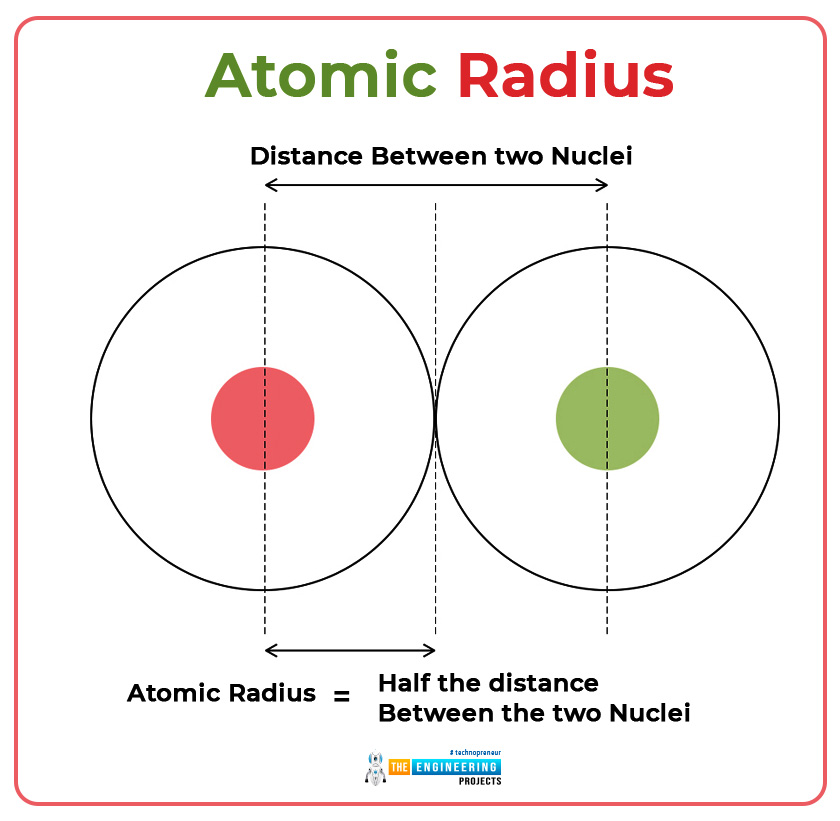
The distance between the nucleus and the outermost orbit of the electrons of an atom is called atomic radius or atomic radii.
Relative atomic mass
The relative mass unit or atomic weight open element is defined as the number of atoms of an element in grams contained in 12 grams of carbon _12(isotope)
Atomic mass unit
The atomic mass unit or Dalton is defined as the one-twelfth of the mass of a carbon atom.
- It is also called Dalton.
- Its symbol is amu.
Interactions between atoms ( bonds)
Types of bonds
- Ionic bond
- Covalent bond
- Dative covalent bond
- Metallic bond
Ionic bond:
This type of bond is formed by the complete transfer of an electron from one atom to another.
Example
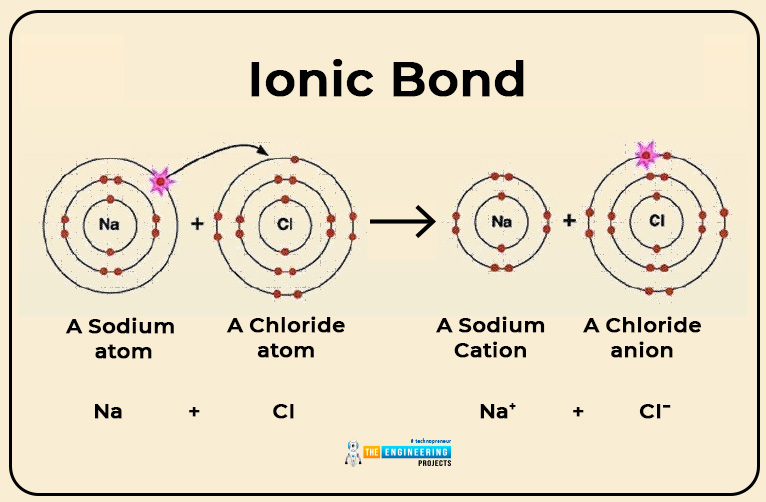
- Only valence electrons( electrons in the last shell) can take part in ionic bonding while others are not.
- In ionic bond formation, heat is released.
Covalent bond:
In this type of bond, electrons are mutually shared between two atoms.
Types
- Single covalent bond
- Double covalent bond
- Triple covalent bond
- Metallic bond
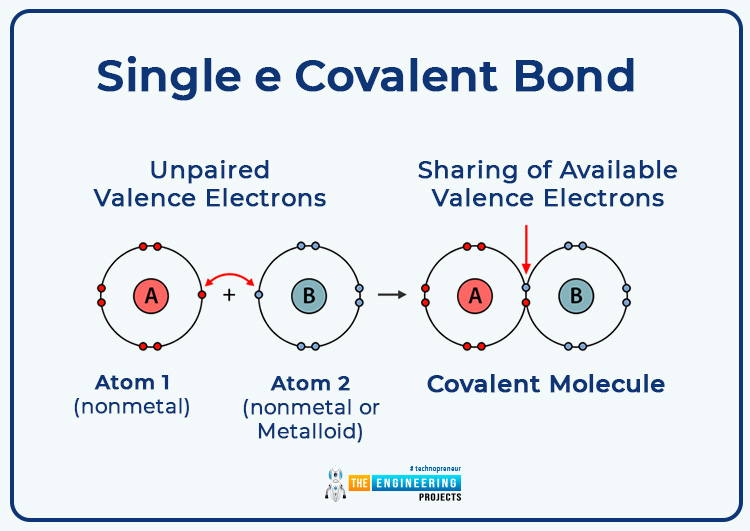
Single e covalent bond:
In which one bond pair of electrons is formed by the contribution of an electron by each bonded atom.
- Indicated by a single line
Double covalent bond:
In which two bond pair is formed by the contribution of electron pair from each atom.
- Indicated by double lines
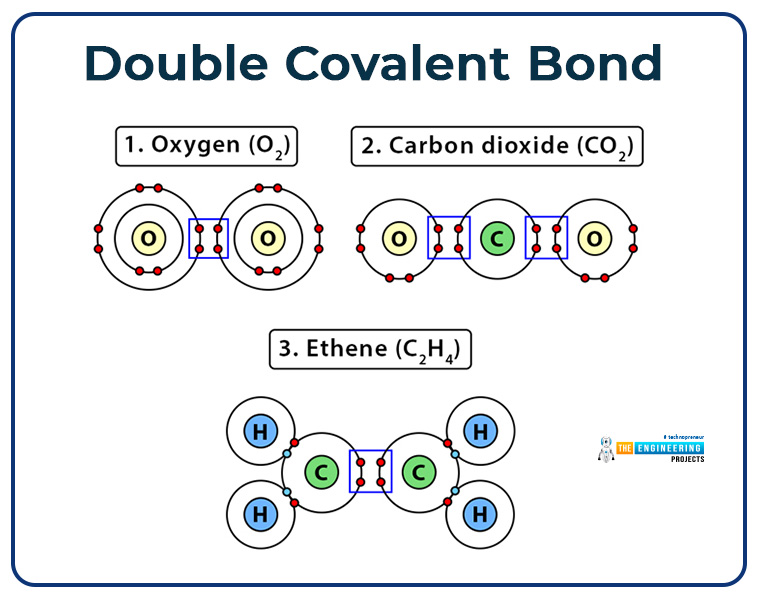
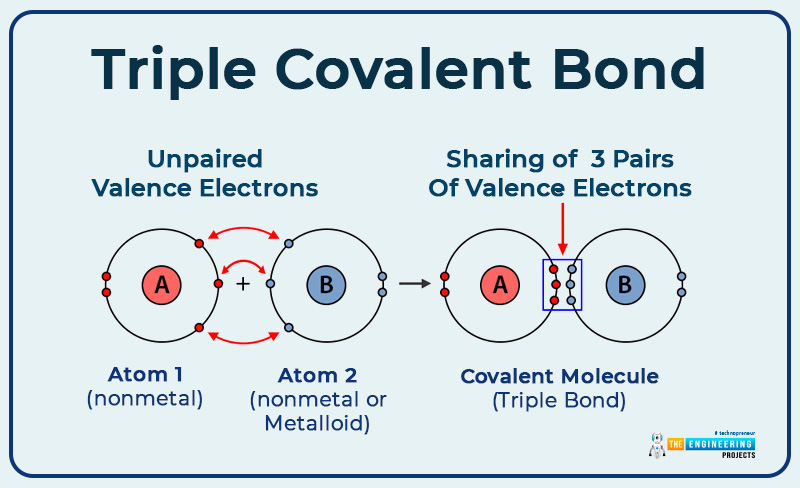
Triple covalent bond:
In which three bond pairs are formed by the contributions of three electrons from each bonded atom.
- It is indicated by three lines.
- Each bonded atom attains octet by sharing of bond pair of electrons and attaining the nearest noble gas configuration.
Dative covalent or coordinate covalent bond:
A bond is formed between the electron pair donor and the electron pair acceptor.
- The atom which invests a pair of electrons is called a donator.
- The atom that receives that electron pair is called the acceptor.
- Those valence electrons that are not taking part in bonding and available on an atom like the one available on nitrogen in ammonia.
Example:
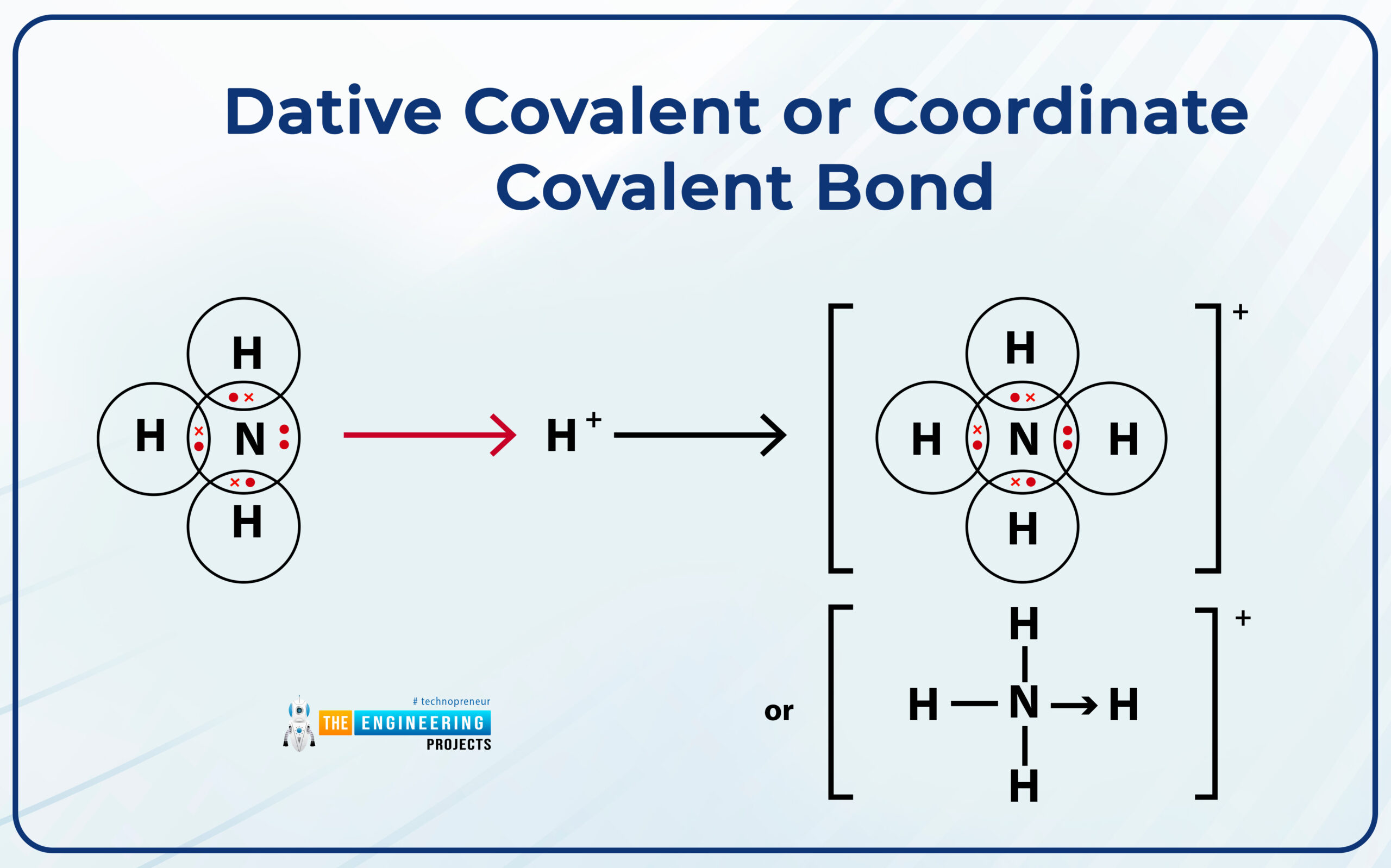
Polar covalent bond:
Those covalent bonds in which hetero atom takes part and one attracts the bond pair of an electron more strongly than the other.
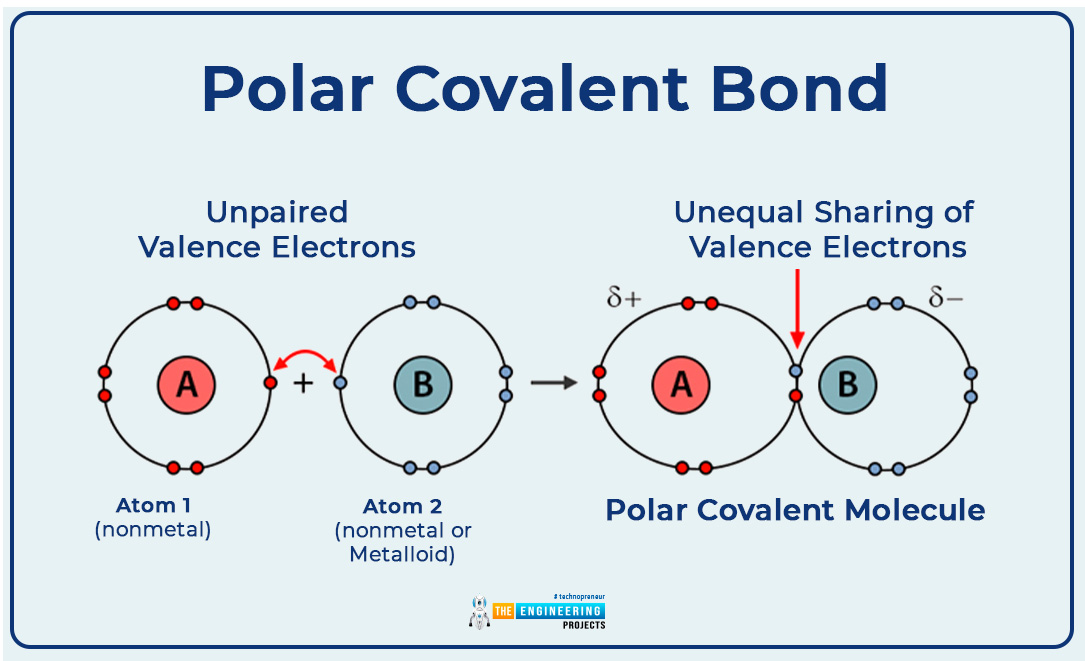
None polar covalent bond:
If a covalent bond is constituted in which two similar atoms shared pair of the electron is excited by the both equally. such a type of bond is called a nonpolar covalent bond.
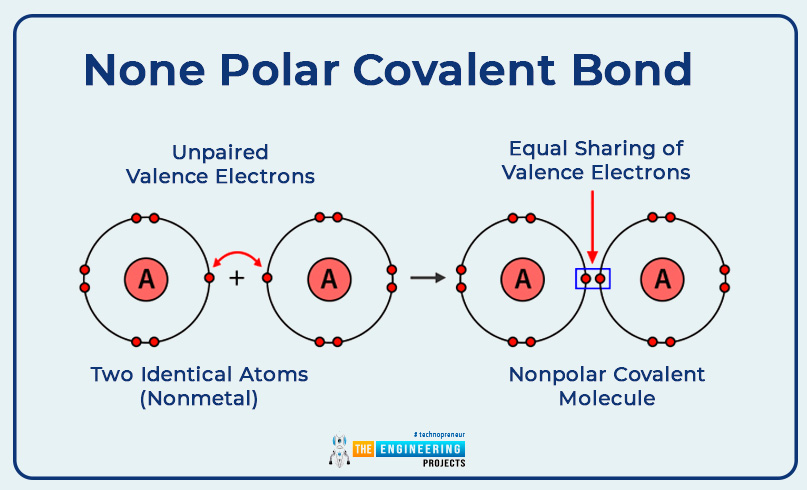
Predominantly covalent:
If the electronegativity between two elements is more than 1.7 the bond between them will be predominantly ionic and if it is less than 1.7 the bond between two atoms will be predominantly covalent.
Metallic bond:
A metallic bond is formed due to free electrons.
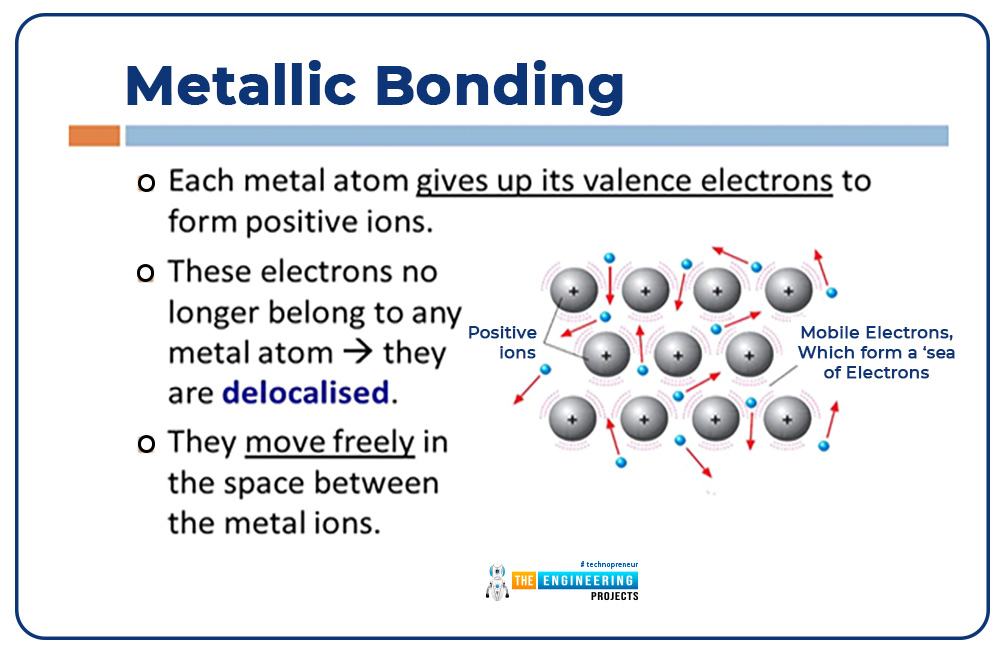
Evolutionary history of the atom

Timeline: 400 BC.
Scientist: Democritus
Theory of universe:
A first-person who uses the term atom(mean individual derived from atoms) was a Greek philosopher. He says that if you divided a piece of matter and divide and continue dividing, at any moment reach when you can’t divide it more, that fundamental unit was Democritus called an atom.
- Atom is made from matter and can not be seen by the necked eye.
- Between atoms, there is a space.
- Atoms are in the form of a solid-state.
- There is no internal structure in an atom.
- Every atom has a different shape, size, and weight.

Timeline:1800s
Scientist: Jhon Dalton
- The first person presents an atomic model of the behavior of the theory of the behavior of atoms consisting of small particles.
- Atoms cannot change their shape and are indestructible.
- By the weight of atoms, their elements can be characterized.
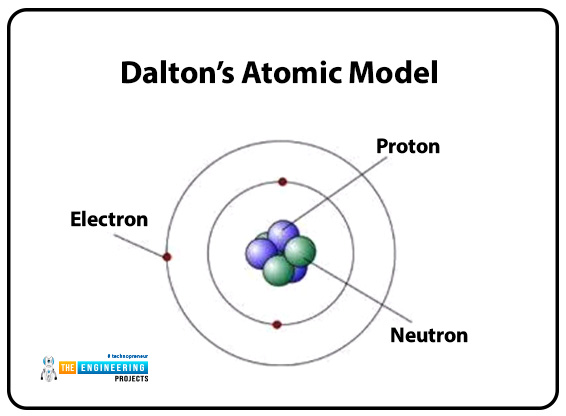
- Atoms combined to firm a compound.

Timeline: 1890s.
Scientist: Thomson
J.JThomson was a physicist who use cathode ray tube technology to discover electrons.
- In this tube, the air has been sucked out.
- An electric charge travels from cathode to anode
- Florescent gas light up the whole tube and the charge in it is invisible. When a charge hits the beam, a dot will appear on the screen.
- A beam of light travel in a straight line in a fluorescent tube.
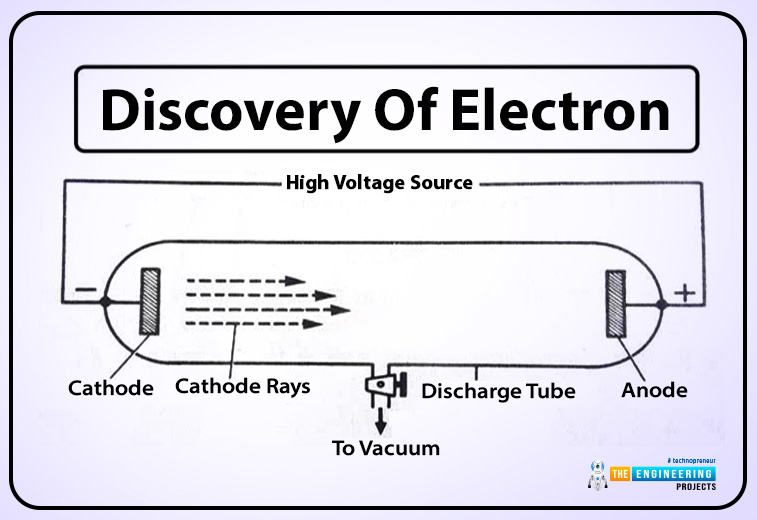
- Each coil has a specific charge on a deflection.
- From a negative coil, the charge would defect away as Thomson shows.
- So he formed that charge this a negative charge.
When he found that negative charge, he did not stop and did a series of experiments, he discover the mass of the electron. He found that the mass of an electron is 1000 times lighter than a hydrogen atom. He made a statement saying that an electron must be inside an atom. Before it, he says the negative charge corpuscles later his name was changed and named as an electron.
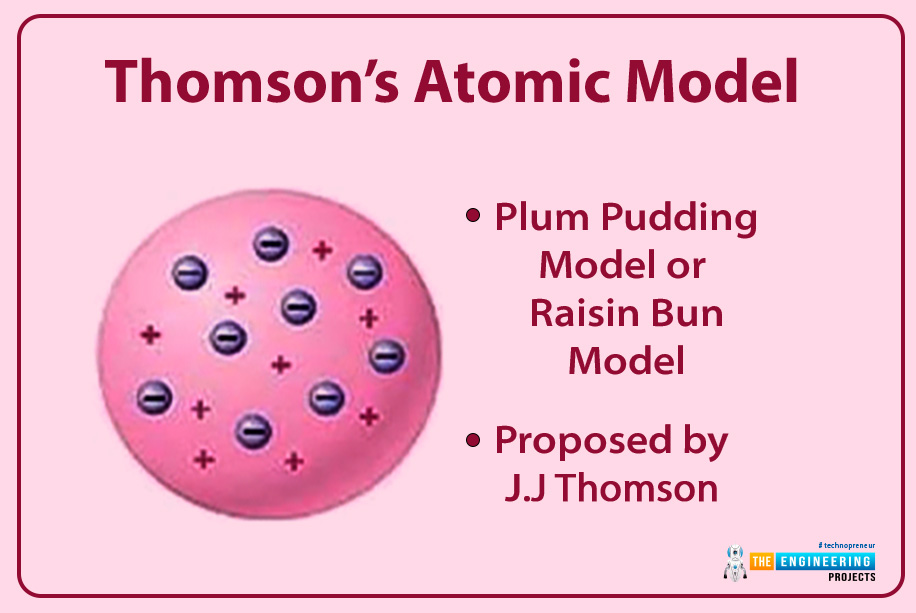
Thomson’s atomic model
Using her prediction, he discovered what an atom looks like?
- His model was named plum pudding model his name.
- Each atom has a spherical shape and is inserted with positively charged fluid. He says it sticky jam part of a pudding.
- In this fluid, corpuscles (electrons) are the negatively charged particles suspended. He compared it to plum in the pudding.
- The movement of these electrons is not discovered by Thomson.

Timeline: 1910’s.
Scientist: Ernest Rutherford
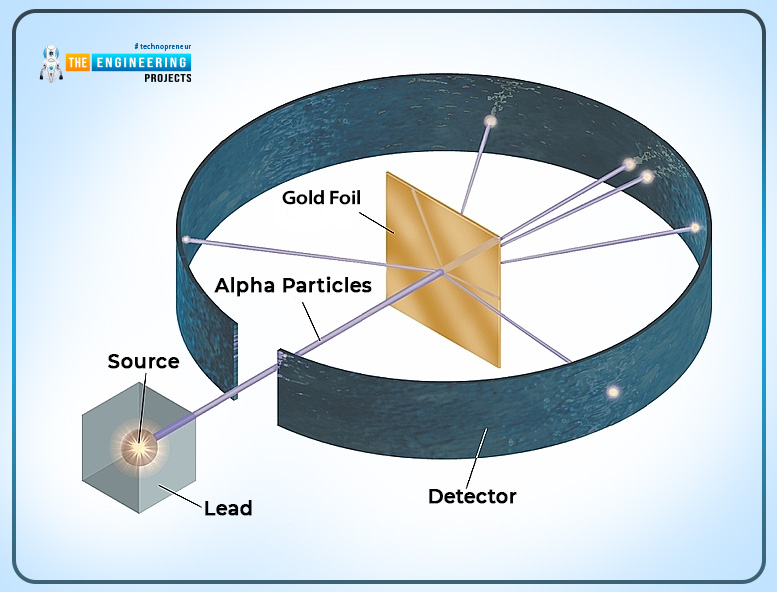
Sir Rutherford made a famous gold foil experiment and proved the Thomson atomic model.
- He fired positively charged alpha particles at a gold foil.
- He estimated the deflection came out the other side.
- Every particle has not been deflected. Every particle would deflect back in all the ways.
- He assumed that the center of the foil must be positive. He called his nucleus.
Rutherford's Atomic model
- The nucleus consists of positively charged particles.
- The electrons revolve around the nucleus.
- He faces one problem: Why nucleus can’t attract the electrons?
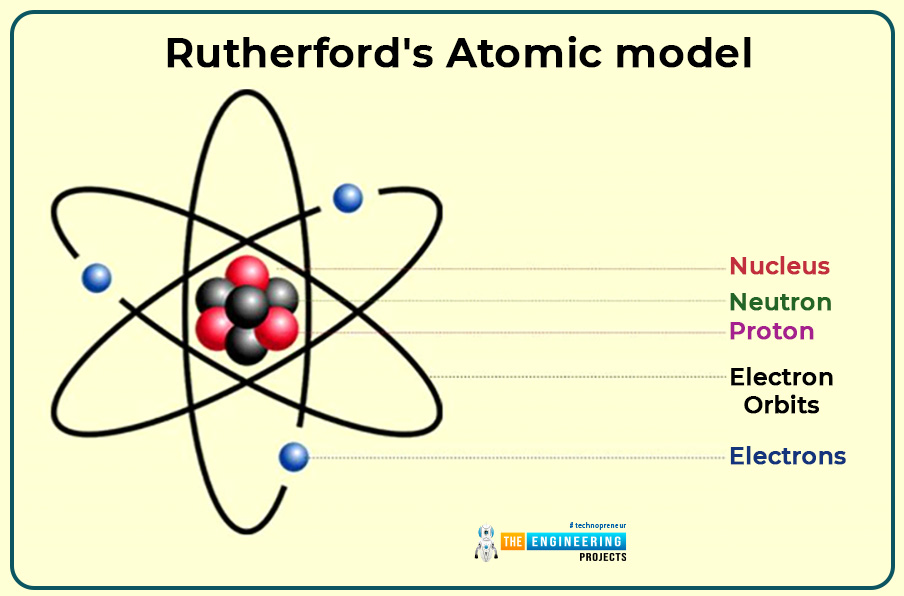
- He compared the atom with a mini solar system in which electrons revolve around the nucleus in a fixed orbit due to this he called his planetary model

Timeline:1910’s
Scientist: Neils Bohr
Niels obeys the planetary model but he found some disadvantages. He could answer the Rutherford question
Why electrons do not fall into the nucleus?
He replies with a perfect answer to the question because of his knowledge of quantum physics and energy.
Bohr's atomic model:
- Electrons have fixed energy and size in orbits of the nucleus.
- The energy of an electron depends on the orbit in which the electron revolves.
- Electrons fill the lower orbit first.
- If the first energy level(orbit) is filled then the next will began to fill.
- When an electron moves from one orbit to another, the radiation process occurs.
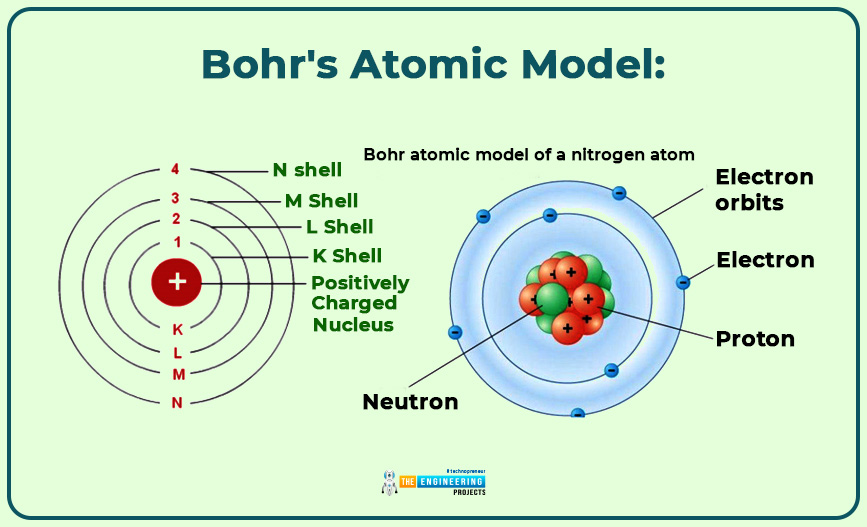
There is a problem with this theory: Electrons could not move in a specific path or orbit.

Timeline:1920’s
Scientist: Erwin Schrodinger
He was a revolutionary physicist and he presented the atomic model by using Heisenberg’s uncertainty principle.
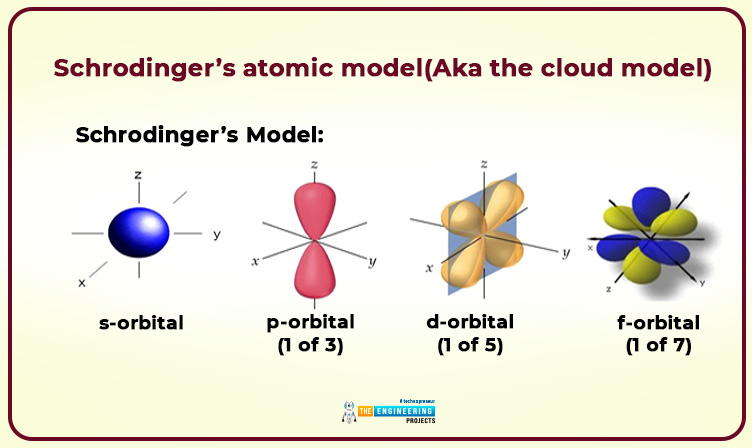
Schrodinger’s atomic model(Aka the cloud model)
- An electron would not revolve in a fixed orbit.
- We can find out where it likely is.
- Energy level depends upon the type of probability of orbit described by Bohr.
Facts
What is not an atom?
By the definition, atoms are the units of matter, so those are not atoms that do not consist of matter.
Parts of atoms that are not associated with a proton are not atoms.
An electron is not an atom, also neutrons bonded to other neutron is not an atom.
Why does an atom react?
Atoms react for attaining the nearest noble gas configuration and become more stable by following the duplet or octet rule.
Why the shape of the atom is spherical?
We can say that an atom “has the shape of the sphere” because a positively charged nucleus is at the very center, and the negatively charged electrons are distributed around it. The electrons are attracted to the nucleus and repel each other. A nucleus, the mass of neutrons and protons within an atom, arranged itself in a roughly spherical shape.
Why the revolving electron does not fall into the nucleus?
Electrons revolve in fixed energy levels around the nucleus. It can not befall into the nucleus because electrons do not radiate energy and move in a circular orbit due to necessary centripetal force.
Why neutrons are neutral and do they exist in the nucleus?
We have an idea from its name, the neutron is the neutron. In other words, the interaction between protons and electrons can cause the formation and destorarion of neutrons. As electrons are negatively charged and protons are positively charged particles. So they cancel each other charge and that’s why neutrons carry no charge and they are neutral. They exist in the nucleus for the stability of nuclei.
Why carbon 12 is used in the references of all atomic mass units?
At the start, hydrogen is used to measure but it gives a fraction. so it would be changed into oxygen, Scientists used a mixture of natural oxygen but it led to confusion. So again changed the reference and turned it into carbon – 12. We use this because it gives all the atomic mass units in exact no. The reason is the different ratio of the mass of proton and neutron performing the change of nuclei.
I Hope, I cover all aspects of the atom, in the next tutorial we will learn about the molecules. If someone has any questions about the atom I will try to answer them. write it in the comment box. Thanks



 Chemistry
Chemistry ahmedyasin
ahmedyasin 0 Comments
0 Comments

















